In September 2025, researchers at the University of Milano published something remarkable in the Journal of Natural Products.
They discovered a cannabinoid that’s been hiding in cannabis plants all along. Cannabizetol. The third known “dimeric cannabinoid” ever found.
So rare—under 0.1% of plant material—that it took specialized extraction and synthesis just to identify it.
But here’s what made me sit up and take notice: When they tested it against inflammation in human skin cells, cannabizetol downregulated 17 inflammatory genes. Its parent molecule, CBG, only downregulated 2.
Not twice the effect. Not even five times. Nearly nine times more genes affected.
This matters for anyone using full-spectrum CBD oils. Because cannabizetol is almost certainly in there. In trace amounts. Contributing to effects we attribute to other cannabinoids.
In this article, I’ll break down:
- What cannabizetol is and how it forms
- The anti-inflammatory research findings (the gene expression data is fascinating)
- Why dimers are dramatically more potent than parent molecules
- Whether it’s in your full-spectrum oil (short answer: yes)
- The synthetic vs natural distinction (why this isn’t “Spice”)
- What this means for the entourage effect
- Study limitations and what we don’t know yet
- What comes next in cannabinoid research
Let’s dive in.
What Is Cannabizetol?
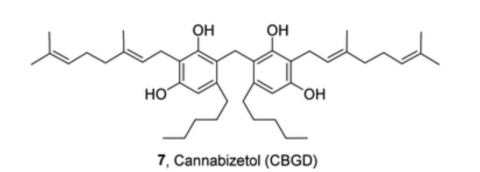
Chemical name: Cannabizetol (abbreviated as CBGD, which stands for CBG Dimer)
Structure: Two cannabigerol (CBG) molecules linked together by a methylene bridge (-CH2-)
Class: Methylene-bridged dimeric cannabinoid (extremely rare)
Discovery: First isolated and fully characterized in 2025
Concentration: Under 0.1% of plant material (approximately 0.02-0.06%)
Understanding Dimers Simply
Imagine two LEGO blocks clicking together.
Each block—let’s say it’s CBG—has certain properties. It fits certain spaces. It connects certain ways.
But when you click two blocks together with a connecting piece (the methylene bridge), the new structure has entirely different properties than either block alone.
It’s bigger. It has a different shape. It connects to things the single blocks couldn’t reach.
That’s what happens with cannabizetol. Two CBG molecules link together through a single carbon atom. That simple connection changes everything about how the molecule behaves.
The Other Known Dimers
Cannabizetol isn’t the first dimeric cannabinoid discovered. It’s the third:
- Cannabisol – A dimer of THC (first discovered)
- Cannabitwinol (CBDD) – A dimer of CBD (discovered in 2020)
- Cannabizetol (CBGD) – A dimer of CBG (discovered in 2025) ← NEW
All three share the same basic structure: two cannabinoid molecules holding hands through a methylene bridge.
Why So Rare?
Dimeric cannabinoids are incredibly rare for several reasons:
Low natural concentrations: They form in tiny amounts—under 0.1% of plant material. That means in 1000mg of cannabinoids, maybe 1mg is cannabizetol.
Difficult to isolate: Extracting and purifying something present at 0.02% requires sophisticated equipment and techniques.
Not routinely tested: Standard cannabinoid testing panels screen for 10-15 major cannabinoids. Dimers aren’t on the list. Labs don’t even have reference standards for them.
Only recently characterized: We didn’t know what to look for. Scientists needed to synthesize cannabizetol first, characterize its structure, then go back to plant extracts to confirm it exists naturally.
How Do They Form?
Dimers can form through several pathways:
- During extraction: Heat, solvents, and processing can trigger dimerization
- During storage: Light, oxygen, and time promote chemical reactions
- Naturally in the plant: May exist in raw plant material in trace amounts
- Aging process: Cannabis naturally ages and transforms over time
If you could see cannabizetol at the molecular level, it would look like two CBG molecules holding hands through a single carbon atom. That’s the methylene bridge. One carbon. But it changes everything.
The Anti-Inflammatory Research
The University of Milano team didn’t just discover cannabizetol. They tested it.
They wanted to know: does this dimer have biological activity? And how does it compare to its parent molecule (CBG) and the other known dimer (cannabitwinol)?
The Study Setup
Cell type: HaCaT cells (human keratinocytes – skin cells)
Why skin cells? These are the most widely used model for studying skin inflammation. They’re involved in conditions like acne, eczema, and psoriasis.
Comparison: They tested three compounds side-by-side:
- CBG (parent monomer)
- Cannabitwinol (CBD dimer)
- Cannabizetol (CBG dimer)
Focus: Inflammatory pathways relevant to skin conditions
Let me walk you through what they found.
Finding #1: IL-8 Inhibition
IL-8 is an inflammatory marker that recruits immune cells to sites of inflammation. When you have acne, eczema, psoriasis, or general skin irritation, IL-8 levels go up.
The researchers induced inflammation with TNFα (a pro-inflammatory signal), then treated cells with cannabizetol.
The results:
At 1 μM concentration: 30% reduction in IL-8
At 5 μM concentration: Complete elimination of IL-8 release
Not reduced. Not suppressed. Completely shut down.
The IC50 (concentration that inhibits 50% of the response) was 1.46 μM for cannabizetol.
For comparison, cannabitwinol (the CBD dimer) had an IC50 of 6.39 μM.
Cannabizetol is 4.4 times more potent than cannabitwinol at shutting down IL-8.
Finding #2: NF-κB Inhibition
NF-κB is the master switch for inflammation.
Think of it like the control panel for your home’s electrical system. Flip the main breaker, and everything downstream turns off.
NF-κB controls hundreds of inflammatory genes. When it’s activated, those genes turn on. When it’s inhibited, they stay quiet.
Cannabizetol inhibited NF-κB activation with an IC50 of 4.95 μM.
Cannabitwinol needed 19.8 μM to achieve the same effect.
Cannabizetol is 4 times more potent at shutting down the master inflammation switch.
Finding #3: Gene Expression Analysis (The Most Impressive)
This is where cannabizetol really surprised the researchers—and me.
They ran a gene expression array testing 84 inflammatory genes. These genes control everything from cytokine production to immune cell recruitment to tissue damage.
They treated cells with TNFα to induce inflammation. Then they added either:
- CBG alone (the parent monomer)
- Cannabizetol (the dimer)
Results with CBG: Only 2 genes were downregulated significantly (CCL5 and CCL2)
Results with cannabizetol: 17 genes were downregulated significantly
Let that sink in. The dimer affected 8.5 times more genes than its parent molecule.
Which genes were affected?
Some of the most important ones:
- CXCL8 (IL-8) – Recruits neutrophils, drives inflammation
- CCL20 – Implicated in psoriasis and inflammatory skin conditions
- CXCL1 – Promotes neutrophil recruitment and inflammatory signaling
- IL-1β – Pro-inflammatory cytokine
- TNFα – Master pro-inflammatory signal
- Plus 12 others involved in skin inflammation pathways
This isn’t just suppressing one pathway. Cannabizetol affects multiple inflammatory pathways simultaneously. A multi-target approach.
Exactly what you want for complex inflammatory conditions.
Finding #4: Antioxidant Activity
Beyond anti-inflammatory effects, cannabizetol showed “remarkable antioxidant” activity.
Higher than cannabitwinol. Higher than expected.
This is important because inflammation and oxidative stress often go hand-in-hand. Inflammatory conditions create oxidative damage. Oxidative damage drives more inflammation. It’s a vicious cycle.
Cannabizetol addresses both problems at once.
Safety Profile
Zero cytotoxicity at all tested concentrations (0.5-20 μM).
This is critical. Potent doesn’t mean toxic. Cannabizetol shuts down inflammation without harming cells.
Why Dimers Are More Potent Than Parents
Here’s what puzzled me when I first read this study:
CBG is already anti-inflammatory. We know this. It’s been tested.
So why is cannabizetol—literally two CBGs linked together—so much more potent?
Shouldn’t it be about twice the effect? Instead, it’s affecting 8.5 times more genes.
The Answer: New Molecular Architecture
When two cannabinoid molecules link through a methylene bridge, you don’t just get “2x cannabinoid.”
You get a completely new molecular structure with new properties.
Different binding geometry: The dimer has a different 3D shape than the monomer. It may fit into receptor binding sites differently. It might access pockets the monomer can’t reach.
New interaction points: The methylene bridge creates additional areas where the molecule can interact with proteins, receptors, and enzymes.
Enhanced selectivity: The dimer might bind more selectively to certain targets while ignoring others.
Think of it like this:
You have two screwdrivers. You duct-tape them together. Now you have… well, an awkward tool that doesn’t work as well as two separate screwdrivers.
But what if you hinge them together properly? Now you have pliers. Same basic components (metal handles), but the hinge lets them do things two separate screwdrivers never could. Grip. Twist. Pull. Entirely new functions.
That’s what the methylene bridge does. It’s not duct tape. It’s a strategic connection that creates new possibilities.
Chemical Space Expansion
Here’s a quote from the researchers:
“Natural dimeric compounds are of considerable importance, as they enable further exploration of chemical space, potentially leading to novel biological activities beyond those of their respective monomers.”
What does “chemical space” mean?
Imagine all possible drug-like molecules plotted on a map. Each point on the map is a unique molecular structure. Some areas of the map are crowded (we’ve explored them). Other areas are empty (we haven’t been there yet).
Dimers let us explore new areas of the map. Areas that monomers can’t reach.
And in those new areas? Potentially entirely new biological activities.
Why This Matters for Cannabis Research
For decades, cannabis research focused on monomers:
- THC (the psychoactive one)
- CBD (the non-psychoactive one)
- CBG (the “mother cannabinoid”)
- CBC, CBN, and others
We’ve studied how these individual compounds work. Their mechanisms. Their effects.
But dimers explore entirely different territory.
Each new dimer represents a new set of properties. New mechanisms. New possibilities.
We’re not just studying 150+ known cannabinoids. We’re studying combinations of those cannabinoids. The math gets big quickly.
The Implication
The cannabis plant isn’t just producing cannabinoids. It’s producing dimers—rare, potent compounds that emerge from base cannabinoids.
And we’re only now starting to find them.
The Synthetic vs Natural Question
Let’s talk about the elephant in the room.
Scientists made cannabizetol in a laboratory.
For many people, “synthetic cannabinoid” triggers immediate fear. And rightfully so.
Spice. K2. Synthetic cannabinoids have caused hospitalizations, psychosis, seizures, and deaths.
But there’s a critical distinction most people don’t understand.
What ARE “Synthetic Cannabinoids”? (The Dangerous Kind)
When news reports talk about “synthetic cannabinoids,” they mean compounds like:
- JWH-018
- AM-2201
- AB-FUBINACA
- Dozens of others with chemical-sounding names
These are:
- Entirely NEW molecules that never existed in nature
- Designed from scratch to bind to cannabinoid receptors
- Created to bypass drug laws (marketed as “legal highs”)
- Sold as “Spice”, “K2”, or other brand names
- Unpredictable effects because they’re untested
- Bind to CB1 receptors 100 times stronger than THC
- Extremely dangerous – they’ve killed people
This is what killed people. This is what sends people to emergency rooms. This is what everyone should fear.
What Is “Synthesizing a Natural Compound”? (Research)
Cannabizetol is completely different.
It already exists in cannabis plants naturally. It’s been there all along.
Scientists made it in a laboratory to study it.
This is like:
- Synthesizing vitamin C (exists in oranges, made in labs for research and supplements)
- Synthesizing aspirin (originally from willow bark, now made in labs)
- Synthesizing vanillin (vanilla flavor – exists in vanilla beans, also made synthetically)
Thousands of natural compounds are synthesized for research and commercial use. It doesn’t make them dangerous. It makes them accessible for study.
Why Did They Synthesize It?
Here’s why researchers made cannabizetol in the lab first:
Problem: Cannabizetol is so rare in plants (under 0.1%) that isolating enough to study would require tons of plant material. You’d need to process 250 kg of cannabis to get 50-150 grams of cannabizetol.
Solution: Synthesis allowed them to:
- Make a pure reference standard – Know exactly what they’re looking for
- Characterize its structure – Use NMR and mass spectrometry to understand it
- Confirm natural occurrence – Compare synthetic to plant extract
- Get enough material – Test biological activity with adequate supply
Once they had the synthetic standard, they went back to cannabis extracts and found it naturally. From 250 kg of plant material, they isolated pure natural cannabizetol.
It matched the synthetic version exactly. Same structure. Same properties.
This proved cannabizetol is a natural plant product.
The Clear Distinction
| Dangerous “Synthetic Cannabinoids” | Research “Synthesized Natural Compound” |
|---|---|
| NEW molecules never in nature | COPYING what nature already makes |
| Spice, K2, designer drugs | Laboratory research tool |
| Designed to get people high | Made to study properties |
| Unknown safety profile | Natural compound with safer baseline |
| Bind 100x stronger than THC | Natural binding affinity |
| Caused deaths and hospitalizations | Research use, safe in studies |
| Bypassing drug laws | Understanding plant chemistry |
The Bottom Line
Your full-spectrum CBD oil contains cannabizetol naturally. It always has.
Scientists didn’t invent it. They didn’t create something new and dangerous. They discovered something that was already there and gave it a name.
Is Cannabizetol in Your Full-Spectrum Oil?
Direct answer: Almost certainly yes, but in tiny trace amounts.
Why It’s There
Your full-spectrum oil contains CBG. Probably 1-5% depending on the extract.
Dimers form when:
- Heat is applied during extraction
- Light exposure during storage
- Oxygen contact over time
- Natural aging of the oil
- In the raw plant before extraction
If you have CBG in your oil, you likely have cannabizetol. The two molecules link together naturally during processing and storage.
The Concentrations
The study found cannabizetol at under 0.1% in plant material. More specifically, they estimated 0.02-0.06%.
In your oil, it’s likely even lower. Perhaps 0.01-0.05% of total cannabinoids.
To put this in perspective:
If your bottle contains 3,000mg of total cannabinoids, cannabizetol might be 0.3-1.5mg total.
If you take a 30mg dose (1mL of 3% oil), you’re getting approximately 0.003-0.015mg of cannabizetol.
That’s trace amounts. Micrograms, not milligrams.
Why You Don’t See It on COAs
Certificate of Analysis (COA) reports show major cannabinoids:
- CBD
- CBG
- CBC
- CBN
- THC
- Maybe a few others
You don’t see cannabizetol listed. Why?
Detection limits: Most labs have detection limits of 0.1% minimum. Cannabizetol is below this threshold.
Standard panels: Testing panels are designed for known, major cannabinoids. Dimers aren’t included.
No reference standards: Until recently, labs didn’t have cannabizetol reference standards to compare against. You can’t test for something if you don’t know what it looks like.
Cost: Testing for rare, minor cannabinoids requires specialized equipment and costs significantly more.
Not required: Regulatory testing doesn’t require dimer analysis.
So cannabizetol is there. Your COA just doesn’t show it.
Does This Matter?
Here’s the honest answer: We don’t know yet.
The study used concentrations of 1-20 μM in cells. We don’t know how that translates to oral dosing.
We don’t know:
- How much cannabizetol is absorbed when taken orally
- How it’s metabolized in the liver
- What concentrations reach tissues
- Whether trace amounts have biological effects
- If other cannabinoids boost its activity (entourage effect)
But here’s what we DO know:
Full-spectrum extracts contain active compounds we don’t routinely test for. This study proves it. Those compounds show remarkable activity in research. And they may be contributing to effects we attribute to major cannabinoids.
The Entourage Effect Implication
When researchers talk about the “entourage effect,” they usually mean:
CBD + THC + CBG + terpenes + flavonoids = better than CBD alone
But this study suggests the entourage effect is far more complex than we thought.
It might actually be:
CBD + THC + CBG (the monomers)
- Cannabitwinol + Cannabizetol (the dimers)
- 50+ other minor cannabinoids
- Terpenes
- Flavonoids
- Compounds we don’t have names for yet
The whole is greater than the sum of parts we can identify.
The Full-Spectrum Iceberg
Think of your Certificate of Analysis as showing you the tip of an iceberg.
What’s visible above water is important. But there’s 10 times more mass below the surface.
Layer 1: Above Water (Visible on COA)
Major cannabinoids:
- CBD, CBG, CBC, CBN, etc.
Concentrations:
- 0.5-30% typically
Testing:
- Standard panels detect these
- Routinely measured
- Well-understood
Research:
- Extensively studied
- Mechanisms known
- Effects characterized
In Dr. Hemp Me oils:
- CBD: 3-30% depending on product
- CBG: 1-5%
- CBC: 0.5-2%
- CBN: 0.1-1%
This is what you see. This is what we talk about. This is what marketing focuses on.
Layer 2: Just Below Surface (Sometimes Tested)
Minor cannabinoids:
- CBDV, THCV, CBT, CBDA, CBGA, etc.
Concentrations:
- 0.1-1% typically
Testing:
- Advanced panels detect some
- Specialized labs
- More expensive
Research:
- Emerging studies
- Some mechanisms understood
- Potential applications being explored
Examples:
- CBDV for epilepsy
- THCV for appetite regulation
- CBT for various effects
Most companies don’t test for these. Some do. They’re there whether you test or not.
Layer 3: Deep Below (Rarely Tested)
Trace cannabinoids:
- Cannabizetol
- Other dimers
- Metabolites
- Degradation products
Concentrations:
- <0.1% (often <0.05%)
Testing:
- Specialized research only
- Requires expensive equipment
- Not commercially available
Research:
- Just being discovered
- Limited understanding
- This study proves this layer exists AND it’s highly potent
Examples:
- Cannabizetol (CBG dimer)
- Cannabitwinol (CBD dimer)
- Cannabisol (THC dimer)
- Unknown dimers
- Unknown metabolites
Layer 4: Ocean Floor (Unknown Territory)
Unidentified compounds:
- No names yet
- Structures unknown
- Properties unknown
Concentrations:
- Unknown (likely <0.05%)
Testing:
- Don’t know what to look for
- Can’t test for what we don’t know exists
Research:
- Waiting to be discovered
- Could be decades of research ahead
Possibilities:
- Dimers of THC+CBD
- Dimers of CBG+CBC
- Trimers (three cannabinoids linked)
- Oxidation products
- Novel structures
The Revelation
This cannabizetol study revealed something profound:
Layer 3 isn’t just there—it’s MORE potent than Layer 1.
Cannabizetol (trace compound, <0.1%) affected 8.5 times more genes than CBG (major compound, 1-5%).
What else is in Layer 3? What’s in Layer 4?
What other rare compounds are contributing effects we attribute to major cannabinoids?
Why We Use Full-Spectrum
This is why Dr. Hemp Me uses full-spectrum extraction.
We don’t just want Layer 1. We want all four layers.
Because science keeps proving the deep layers matter.
Isolate CBD = Layer 1 only (actually, just one compound from Layer 1)
Broad-spectrum = Layers 1 and 2 mostly
Full-spectrum = All four layers, including the parts we can’t test for
Study Limitations & What We Don’t Know
Every study has limitations. Here’s what this cannabizetol study can—and can’t—tell us.
Limitation #1: Cell Studies Only
What they did:
- Tested in keratinocytes (skin cells)
- Cells grown in plastic dishes
- Controlled laboratory conditions
What this means:
- Cells in dishes don’t behave exactly like cells in bodies
- Missing immune system interactions
- Missing blood flow, tissue complexity
- Missing whole-organism effects
What we need:
- Animal studies (mice, rats)
- Eventually human trials
- Real-world data
Limitation #2: The Concentration Question
What they used:
- 1-20 μM concentrations in cells
- Direct application to cells
What we don’t know:
- How much you’d need to take orally to reach these concentrations in skin
- Whether cannabizetol survives digestion
- How liver metabolism affects it
- What concentrations reach tissues
- Bioavailability from oral consumption
What we need:
- Pharmacokinetic studies (absorption, distribution, metabolism)
- Dose-response studies in animals
- Human dosing trials
Limitation #3: Skin-Specific Testing
What they tested:
- Human keratinocytes only
- Skin inflammation markers
What we don’t know:
- Does it work in other tissues?
- What about gut inflammation?
- What about joint inflammation?
- What about neuroinflammation?
- Skin ≠ systemic inflammation
What we need:
- Multi-tissue testing
- Different cell types
- Different inflammatory models
Limitation #4: Trace Amounts in Products
The reality:
- Natural oils contain ~0.01-0.05% cannabizetol
- Study used pure cannabizetol
- Huge concentration difference
The questions:
- Do trace amounts matter clinically?
- Do other cannabinoids boost its effect?
- Is there a threshold for activity?
- Does chronic low-dose exposure work differently?
What we need:
- Studies testing actual full-spectrum extracts
- Comparison of isolated vs trace amounts
- Entourage effect research
Limitation #5: No Long-Term Data
What they tested:
- 6-hour treatment
- Short-term effects
- Safety over hours
What we don’t know:
- Long-term safety
- Chronic exposure effects
- Cumulative effects
- Tolerance development
What we need:
- Long-term safety studies
- Chronic dosing studies
- Human data over months/years
The Realistic View
This study is a first step. An important one.
It proves:
- Cannabizetol exists
- It’s potent in cell studies
- It’s in full-spectrum extracts
- Dimers are more active than expected
But we can’t claim:
- It’s why full-spectrum oils help with skin conditions
- Trace amounts are therapeutically relevant
- It works the same way in humans
- Oral consumption delivers active concentrations
More research is needed. A lot more.
What We CAN Say
We can say full-spectrum extracts are more complex than standard testing reveals.
We can say rare cannabinoids show remarkable activity in research.
We can say the entourage effect is real—just more complicated than we understood.
And we can say science is just beginning to understand what’s in these plants.
What Comes Next: Future Research
The researchers ended their study with this statement:
“These results suggest that among the many still unknown cannabinoids there are also methylene-bridged dimers of other cannabinoids, including dimers composed of two different cannabinoids, with potential biological activities of great interest.”
Let that sink in.
The Possibilities
If cannabizetol (CBG dimer) is this potent, what about:
Same-cannabinoid dimers:
- THC + THC
- CBD + CBD (already found: cannabitwinol)
- CBC + CBC
- CBN + CBN
- CBDV + CBDV
Mixed dimers (two different cannabinoids):
- THC + CBD
- CBD + CBG
- CBG + CBC
- THC + CBN
- Any combination of 150+ known cannabinoids
Triple linkages (trimers):
- Three cannabinoids linked together
- Even rarer
- Potentially even more unique properties
The math gets big quickly.
If there are 150 known cannabinoids, there are potentially:
- 150 homodimers (same + same)
- 11,175 heterodimers (different + different)
- Plus trimers, tetramers, and higher orders
Each one could have unique properties.
The Discovery Timeline
Let’s look at the timeline:
- Cannabisol (THC dimer) – Discovered 2012
- Cannabitwinol (CBD dimer) – Discovered 2020
- Cannabizetol (CBG dimer) – Discovered 2025
We’re finding approximately one dimer every 4-8 years.
How many are still hidden? Dozens? Hundreds?
What Researchers Need
To accelerate discovery:
- Better extraction techniques – Isolate rare compounds more efficiently
- Synthesis programs – Make potential dimers to create reference standards
- Biological screening – Test each dimer for activity
- Structure-activity studies – Understand which structures do what
- Eventually, human trials – Test the promising ones in people
This is decades of work.
Dr. Hemp Me’s Commitment
We’ll keep following this research.
When new dimers are discovered, we’ll break down the studies. When human trials begin, we’ll share the results. When understanding evolves, we’ll evolve with it.
Science moves slowly. But it moves.
What This Means for Full-Spectrum Users
Let’s bring this back to practical reality.
What’s Certain
✅ Your full-spectrum oil contains more than what’s tested
✅ Cannabizetol is almost certainly present in trace amounts
✅ Other dimers are probably present too
✅ Unknown compounds are definitely present
✅ The entourage effect is real and complex
✅ Full-spectrum preserves these trace compounds
What’s Uncertain
❓ Whether trace amounts matter clinically
❓ How cannabizetol is absorbed and metabolized
❓ Whether it works in humans like it does in cells
❓ What other rare compounds are doing
❓ Optimal ratios and synergies
❓ Long-term effects
The Full-Spectrum Advantage
Here’s why this study reinforces full-spectrum:
Isolate approach:
- Extract CBD only
- Miss 149+ other cannabinoids
- Miss all dimers
- Miss all unknown compounds
- Miss entourage effect
- Get pure CBD, nothing else
Full-spectrum approach:
- Extract everything
- Keep major cannabinoids (Layer 1)
- Keep minor cannabinoids (Layer 2)
- Keep trace cannabinoids like cannabizetol (Layer 3)
- Keep unknown compounds (Layer 4)
- Preserve all interactions
- Preserve entourage effect
You’re getting all four layers of the iceberg.
Quality Matters
Not all full-spectrum extracts are equal.
Extraction method matters:
- Harsh extraction can destroy delicate compounds
- High heat can degrade cannabinoids
- Improper techniques lose minor compounds
Storage matters:
- Light exposure degrades cannabinoids
- Oxygen exposure causes oxidation
- Heat accelerates degradation
- Proper storage preserves the profile
Testing matters:
- Verifies cannabinoid content
- Ensures no heavy metals
- Confirms no pesticides
- Guarantees quality